Follicle Growth and Development
Authors
INTRODUCTION
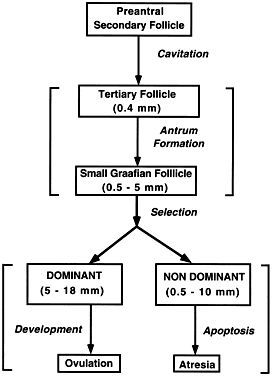
Typically, the human ovaries produce a single dominant follicle that results in a single ovulation each menstrual cycle (Fig. 1). In any given cycle, the dominant follicle must complete all the steps in folliculogenesis in a timely manner. In this capacity, it survives the negative events that operate to destroy the other follicles by atresia. Recognition that only a few follicles become dominant beautifully demonstrates the fundamental principle that folliculogenesis in mammals is a highly selective process. This chapter considers what is known about the process underlying the expression of the structural and functional organization of developing follicles and how they are controlled.
FOLLICULOGENESIS
Folliculogenesis is the process in which a recruited primordial follicle grows and develops into a specialized graafian follicle with the potential to either ovulate its egg into the oviduct at mid-cycle to be fertilized or to die by atresia. In women, the process is long, requiring almost 1 year for a primordial follicle to grow and develop to the ovulatory stage. During the course of folliculogenesis, growth is achieved by cell proliferation and formation of follicular fluid, whereas development involves cytodifferentiation of all the cells and tissues in the follicle. Only a few follicles in the human ovary survive to complete the cytodifferentiation process, with 99.9% dying by a programmed cell death mechanism called apoptosis.
The mechanisms regulating follicle growth and development are under the control of changing concentrations of ligands (i.e. hormones and growth factors). At the endocrine level, folliculogenesis is regulated by a central nervous system, anterior pituitary, and ovary cascade mechanism. Specialized hypothalamic neurons secrete pulses of gonadotropin-releasing hormone (GnRH) into the portal blood vessels, which acts on the gonadotrophs to cause a pulsatile release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which act on ovarian follicle cells to control folliculogenesis. Although GnRH, FSH, and LH are critically important in regulating folliculogenesis, hormones and growth factors, which are themselves products of the follicle, can act locally to modulate (amplify or attenuate) FSH and LH action. This is the autocrine/paracrine system of developing follicles. It is believed that this local regulatory system plays an important role in the complex mechanisms governing the timing of folliculogenesis and whether a follicle becomes dominant or atretic.
Chronology
The steps and timing of human folliculogenesis are shown in Fig. 2. In women, folliculogenesis is a long process.1,2,3 In each menstrual cycle, the dominant follicle that ovulates its egg originates from a primordial follicle that was recruited to initiate growth almost 1 year earlier (Fig. 2). In a broad sense, there are two types of follicles (Fig. 2): preantral (primordial, primary, secondary [class 1], tertiary [class 2]) and antral (graafian, small [class 3, 4, 5], medium [class 6], large [class 7], preovulatory [class 8]). The development of preantral and antral follicles is gonadotropin independent and gonadotropin dependent, respectively.
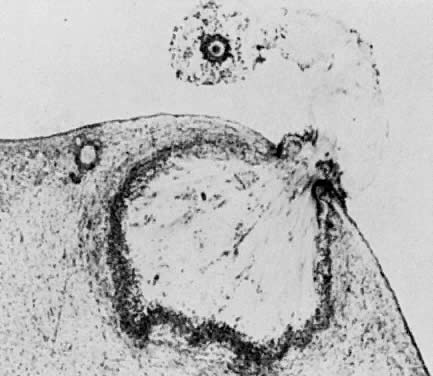
The rate of preantral follicle development is slow, requiring about 300 days for a recruited primordial follicle to complete the whole preantral period (Fig. 2). A long doubling time (about 10 days) for the granulosa cells is responsible for the slow growth rate. After antrum formation in the class 3 follicle (about 0.4 mm in diameter), the rate of growth accelerates (Fig. 2). The time interval between antrum formation and the development of a 20-mm preovulatory follicle is about 50 days (Fig. 2). The dominant follicle appears to be selected from a cohort of class 5 follicles at the end of the luteal phase of the menstrual cycle.1,2,3,4 About 15 to 20 days are required for a dominant follicle to grow and develop to the preovulatory stage (Fig. 2). Atresia can occur in all follicles (preantral and antral) after the class 1 or secondary follicle stage; however, the highest incidence is seen in the antral follicles that are more than 2 mm in diameter (i.e. class 5, 6, and 7) (Fig. 2).
The Process
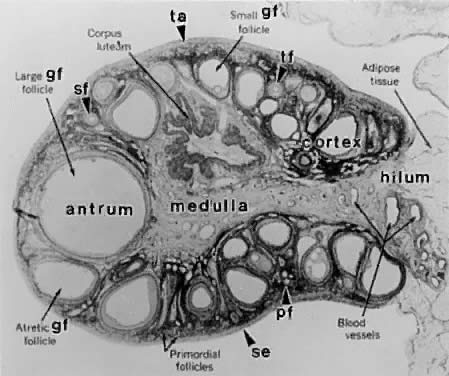
Folliculogenesis occurs within the cortex of the ovary (Fig. 3). The follicles in the cortex are present in a wide range of sizes representing various stages of folliculogenesis. The goal of folliculogenesis is to produce a single dominant follicle from a pool of growing follicles. There are four major regulatory events involved in this process: recruitment, preantral follicle development, selection, and atresia.
THE PRIMORDIAL FOLLICLE.
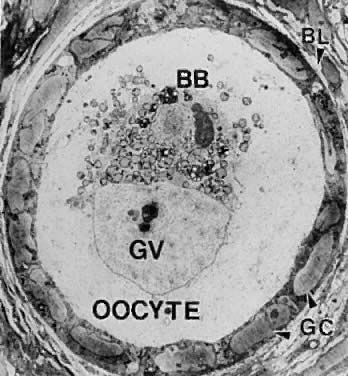
All primordial follicles are composed of a small primary oocyte (about 25 μm in diameter) arrested in the diplotene (or dictyate) stage of meiosis, a single layer of flattened (squamous) granulosa cells, and a basal lamina (Fig. 4). The mean diameter of the human primordial follicle is 29 μm.5 By virtue of the basal lamina, the granulosa and oocyte exist within a microenvironment in which direct contact with other cells does not occur. The primordial follicles do not have an independent blood supply.6 It follows that primordial follicles have limited access to the endocrine system.
Recruitment.
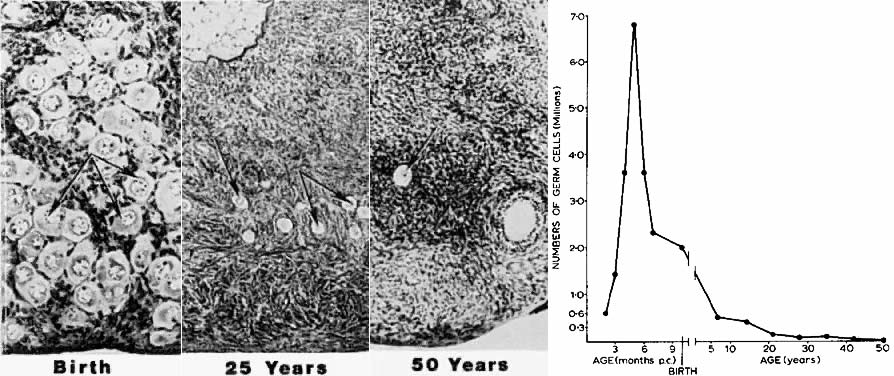
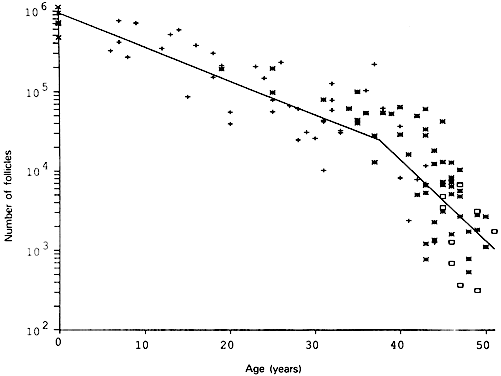
The first major event in folliculogenesis is recruitment. Recruitment is the process by which an arrested primordial follicle is triggered to reinitiate development and enter the pool of growing follicles. All primordial follicles (oocytes) present in the human ovaries are formed in the fetus between the sixth and the ninth month of gestation. Because the entire stock of oocytes in primordial follicles is in meiotic prophase, none is capable of dividing mitotically. All oocytes (primordial follicles) capable of participating in reproduction during a woman's life are present in the ovaries at birth (Fig. 5). The total number of primordial follicles in the ovaries at any given moment of time is called the ovary reserve (OR).7 The process of recruitment begins soon after the formation of the primordial follicles in the fetus,8 and it continues throughout the life of the female until the pool of primordial follicles is exhausted at the menopause (Fig. 5). There is a bi-exponential decrease in OR during aging7,9,10 (Fig. 6). The number of primordial follicles falls steadily for more than three decades, but when the OR reaches a critical number of about 25,000 at 37.5 ± 1.2 years of age, the rate of loss of primordial follicles accelerates about twofold (Fig. 6). This change in OR is associated in an age-related decrease in fecundity, perhaps causal to the age-related increase in FSH that occurs in women after 36 years of age.7
Mechanism.
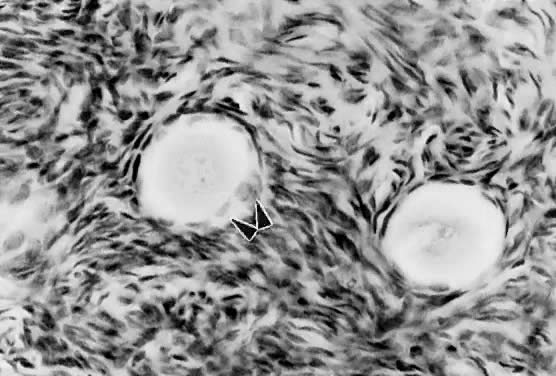
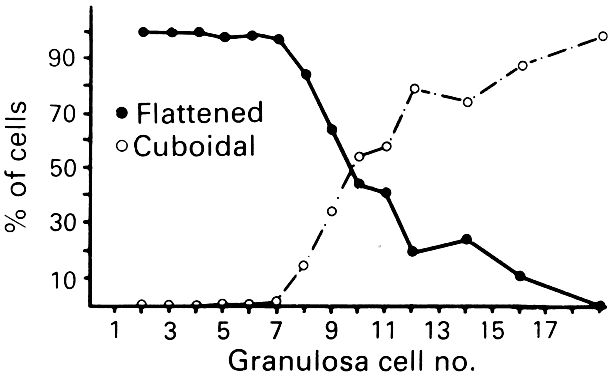
The first visible sign (Fig. 7) that a primordial follicle is being recruited is that some granulosa cells begin to change from a squamous to a cuboidal shape.5 The first cuboidal cell is seen when the primordial follicle contains 8 granulosa cells, and the process is complete when the granulosa number reaches 19 (Fig. 8). The shape change is followed by the onset, albeit slow, of DNA synthesis and mitosis in the granulosa cells.8 A change in shape and acquisition of mitotic potential in the granulosa cells are the hallmarks of recruitment. Such observations suggest that the mechanisms governing recruitment may involve a regulatory response at the level of the granulosa cell. Recruitment is pituitary independent, and it probably is controlled by autocrine/paracrine mechanisms. Whether it is effected by a stimulator or the loss of an inhibitor is uncertain; however, primordial follicles undergo rapid recruitment when removed from the ovary and cultured in vitro.11 These observations support the inhibitor idea.
Several different hypotheses have been put forth to explain the mechanism of recruitment. First, the process appears to occur in primordial follicles nearest the medulla where blood vessels are prominent. This supports the hypothesis that exposure to nutrients or blood-borne regulatory molecules could play a role in the control of recruitment. Second, an internal oocyte clock mechanism has been proposed to control recruitment.12 In this hypothesis, the clock is related to the time that the oocyte initiates meiosis in the embryo. It is noteworthy that recruitment can be modulated.8 In rodents, the rate of recruitment can be attenuated by removing the neonatal thymus gland, starvation, or treatment with exogenous opioid peptides. These are important observations, because they argue that ligand-receptor signaling pathways are likely to regulate recruitment. Understanding the regulatory mechanisms underlying recruitment remains a major task in reproductive biology.
THE PREANTRAL FOLLICLE.
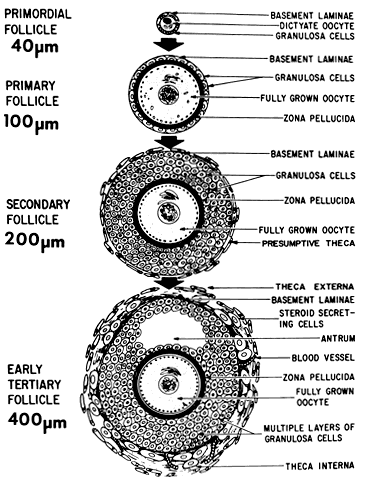
The early stages of folliculogenesis can be divided into three classes based on the number of layers of granulosa cells, the development of theca tissue, and the expression of a small cavity or antrum. The classes are the primary, secondary, and early tertiary follicles (Fig. 9). As the morphologic complexity increases, important cellular and physiologic changes occur in the follicle that render it competent to respond to gonadotropins. The following sections examine the structure and function changes that accompany preantral follicle growth and development.
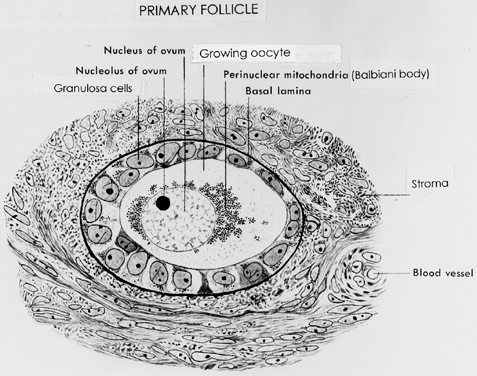
Primary Follicle.
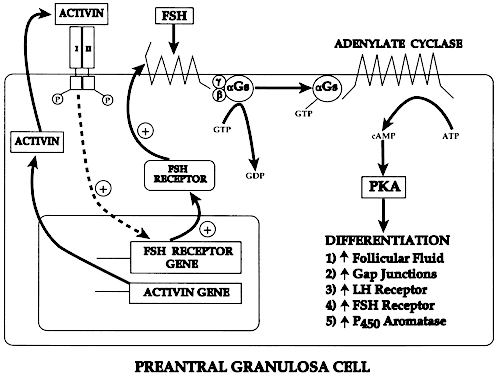
A primary follicle consists of one or more cuboidal granulosa cells that are arranged in a single layer surrounding the oocyte (Fig. 10). Simultaneous with the shape change and mitotic activities that accompany recruitment (Figs. 7 and 10), the cuboidal granulosa cells begin to express FSH receptors.13,14 The mechanism underlying this critical event in folliculogenesis remains uncertain, but there is evidence in rodents15 that granulosa-derived activin may play an important role in the expression of FSH receptor by autocrine/paracrine mechanisms (Fig. 11). Although the granulosa cells express FSH receptors at this very early stage in folliculogenesis, it is believed that the physiologic levels of plasma FSH during the normal menstrual cycle do not influence granulosa responses because primary follicles lack an independent vascular system. Nevertheless, because there are blood vessels in the vicinity (Fig. 10), FSH-induced changes in primary follicle function may occur in response to abnormally high levels of plasma FSH, such as those that occur during ovulation induction or aging.
Beginning approximately at the time of recruitment, the oocyte begins to grow and differentiate. This period is marked by a progressive increase in the level of oocyte RNA synthesis.16 A number of important oocyte genes are turned on at this time. For example, the genes encoding the zona pellucida (ZP) proteins (i.e. ZP-1, ZP-2, and ZP-3) are transcribed and translated.17 The secreted ZP proteins begin to polymerize near the oocyte surface, forming an extracellular matrix coat (the zona pellucida) that eventually encapsulates the egg. The importance of the zona pellucida is emphasized by the fact that the carbohydrate moiety of ZP-3 is the species-specific sperm-binding molecule.18 It is responsible for initiating the acrosome reaction in capacitated sperm.19
During primary follicle development, the granulosa cells send processes through the zona layer, where they form gap junctions with the oocyte cell membrane, or oolemma (Fig. 12). Gap junctions are intercellular channels composed of proteins called connexins.20,21 There are at least 13 members of the connexin family that directly couple adjacent cells to allow the diffusion of ions, metabolites, and other low-molecular-weight signaling molecules such as cAMP and calcium.20,21 Connexin 37 (C×37) is an oocyte-derived connexin that forms gap junctions between the oocyte and surrounding granulosa cells.22 Evidence from C×37-deficient mice assigns C×37 an obligatory role for folliculogenesis, ovulation, and fertility.22 Large gap junctions are also present between the granulosa cells themselves (Fig. 12). C×43 is a major gap junction protein expressed in the granulosa cells.23 As a consequence of gap junctions, the primary follicle becomes a metabolically and electrically coupled unit. This communication between the granulosa and oocyte remains throughout folliculogenesis and is responsible for the synchronous expression of important activities (positive and negative).
Secondary Follicle.
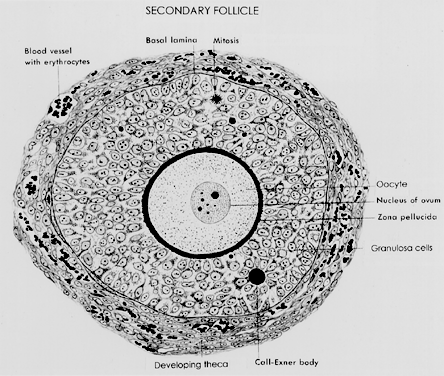
A secondary follicle is a preantral follicle with 2 to 10 layers of cuboidal or low columnar cells that form a stratified epithelium (Fig. 13). As seen in Figure 10, the transition from a primary to a secondary follicle involves the acquisition of a second layer of granulosa cells. This transition is accomplished by the continuing division of the granulosa cells. The mechanisms regulating granulosa mitosis are poorly understood. However, exciting research in rodents has provided compelling evidence for the involvement of an oocytederived growth factor, called growth differentiation factor-9 (GDF-9). GDF-9 is a novel member of the transforming growth factor-β (TGF-β) superfamily.24 GDF-9 is strongly expressed in the ovary; it is localized only in oocytes of recruited follicles.25 In GDF-9 deficient mice, follicle growth and development stop at the primary stage; consequently no dominant follicles form, and the females are infertile.26 Accordingly, GDF-9 is obligatory for folliculogenesis after the primary stage, presumably because it is an obligatory mitogen for granulosa cells. A fundamental concept that emerges from this work is that the oocyte plays a pivotal role in regulating folliculogenesis through its ability to produce novel regulatory ligands (e.g. GDF-9), which are crucial for folliculogenesis.
One of the most important changes that occur in the development of a secondary follicle is the acquisition of a theca layer. This tissue, which consists of a layer of stroma-like cells around the basal lamina, subsequently differentiates into the inner theca interna and outer theca externa (Fig. 13). Theca development is accompanied by the neoformation of numerous small vessels, presumably through angiogenesis (Fig. 13). This is a critical event because blood circulates around the follicle, bringing nutrients and hormones (e.g. FSH, LH) to and waste and secretory products from the secondary follicle. In this regard, some stromal cells in the inner layer express LH receptors.27 These cells subsequently differentiate into steroidogenic cells called theca interstitial cells (TICs), most likely in response to the plasma LH delivered by the theca vascular system.27 All the granulosa cells in secondary follicles express FSH receptors.13 It seems likely that diffusion of plasma FSH into the secondary follicle may evoke FSH-dependent granulosa responses. The outer layer of stroma cells subsequently differentiates into smooth muscle cells called the theca externa. These smooth muscle cells are innervated by the autonomic nervous system.27
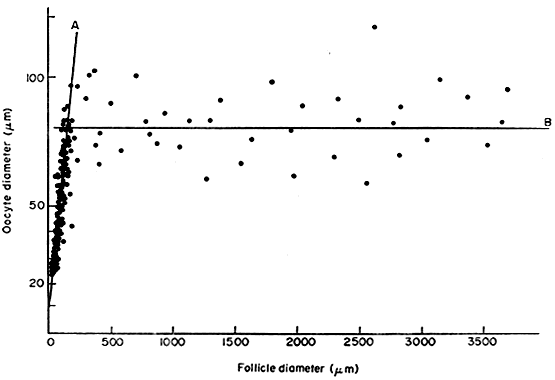
In the secondary follicle, the oocyte completes its growth. When the follicle is about 200 μm in diameter, the oocyte has attained its maximum size and grows no more, despite the fact that the human follicle enlarges to a diameter of 2 cm or more (Fig. 14). It is well documented in rodents that granulosa cells play an obligatory role in the growth and differentiation of the oocyte.28,29 An important differentiation event that occurs when the oocyte completes its growth is acquisition of the capacity to resume meiosis.30 Oocytes normally do not resume meiosis during folliculogenesis, and a mechanism must operate to inhibit this process (i.e. germinal vesicle breakdown [GVBD]) and the resumption of meiosis. The underlying mechanism for the inhibition remains unknown; however, there is evidence to support the concept that granulosa derived cAMP may play an important role in inhibiting the resumption of meiosis.30 In such a mechanism, FSH-induces cAMP in the granulosa cells, which diffuses into the oocyte through the C×37 gap junction, where it proceeds to inhibit GVBD (Fig. 15).
Tertiary Follicle.
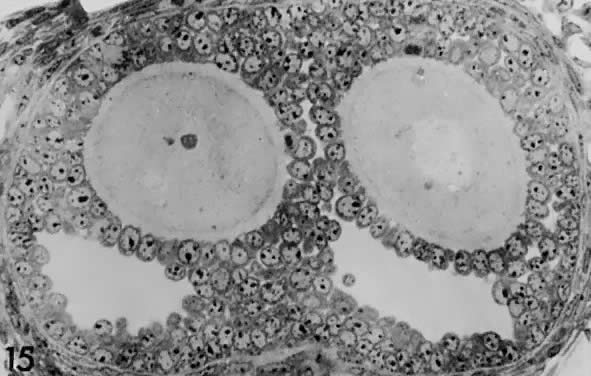
When a preantral follicle completes the secondary stage in development, it contains five distinct structural units: a fully grown oocyte surrounded by a zona pellucida, six to nine layers of granulosa cells, a basal lamina, a theca interna, and a theca externa (Fig. 13). The first indication of the onset of tertiary follicle development is the appearance of a cavity in the granulosa cells.31 In response to an intrinsic stimulus, a cavity begins to form at one pole of the oocyte. This process, called cavitation or beginning antrum formation, is characterized by the accumulation of fluid between the granulosa cells that in time results in the formation of an internal cavity (Fig. 16). At completion of cavitation, the basic plan of the graafian follicle is established, and all the various cell types are in their proper position awaiting the stimuli that will shift them along paths of differentiation and proliferation (Fig. 16). Based on evidence from polyoocyte follicles, the specification mechanism of cavitation probably is tightly regulated (Fig. 17).
What controls cavitation or early antrum formation? It is well known that cavitation occurs in hypophysectomized animals, demonstrating that pituitary hormones such as FSH are not required for this morphogenetic event.32 Consistent with this concept is the observation that cavitation occurs in FSH-β-deficient mice.33,34 It seems reasonable to conclude that cavitation is controlled by autocrine/paracrine mechanisms. Two growth factors expressed in the follicle itself have been implicated in cavitation: activin and KIT ligand. Treating cultured granulosa cells with activin causes morphogenetic changes that result in the formation of a histologic unit with an antrum-like cavity.35 Blocking the action of the KIT ligand in the ovary prevents the formation of antral follicles; consequently, there are no ovulations, and the female is infertile.36 In this regard, evidence supports the concept that the oocyte gap junctions are also important for cavitation. Gap junctions are intercellular channels composed of proteins called connexins.20,21 There are at least 13 members of the connexin family that directly couple adjacent cells, allowing diffusion of ions, metabolites, and other low-molecular-weight signaling molecules such as cAMP.20,21 C×37 appears to be an oocyte-derived connexin that forms gap junctions between the oocyte and surrounding granulosa cells. Evidence from C×37-deficient mice assigns to C×37 an obligatory role in graafian follicle formation, ovulation, and fertility.22 Collectively, all this evidence suggests that follicle-derived activin, KIT, and C×37 are involved in the autocrine/paracrine mechanisms that control cavitation.
THE GRAAFIAN FOLLICLE.
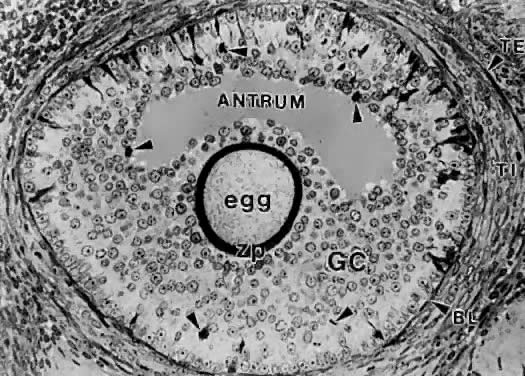
A graafian follicle can be defined structurally as a heterogeneous family of relatively large follicles (0.4 to 23 mm) characterized by a cavity or antrum containing a fluid called follicular fluid or liquor folliculi. The characteristic structural unit of all graafian follicle is the antrum. For this reason, the term antral follicle is used correctly as a synonym for graafian follicle. The follicular fluid is the medium in which the granulosa cells and oocyte are found and through which regulatory molecules must pass on their way to and from this microenvironment.37 Surprisingly, we know almost nothing about the physiologic significance of the antrum and follicular fluid in folliculogenesis. It is clear that follicle development and ovulation occur in birds and amphibians despite the absence of an antrum and follicular fluid. Nonetheless, its presence in all mammalian species testifies to its physiologic importance.
Structure.
A graafian follicle is a three-dimensional structure with a central antrum surrounded by a variety of different cell types (Fig. 18). There are six distinct histologic components in the graafian follicle, including the theca externa, theca interna, basal lamina, granulosa cells, oocyte, and follicular fluid (Fig. 18). A graafian follicle does not change its morphologic complexity as growth proceeds. All graafian follicles have this same basic architecture; even though there are dramatic changes in graafian follicle size, their appearance remains more or less the same.
The theca externa (Fig. 19) is characterized by the presence of smooth muscle cells,38,39 which are innervated by autonomic nerves.27 Although the physiologic significance of the theca externa remains unclear, there is evidence that it contracts during ovulation and atresia.40,41 Changes in the contractile activity of the theca externa may be involved in atresia and ovulation; however, this has not been rigorously proved. The corpus luteum retains a theca externa throughout its life,42 but the significance during luteinization and luteolysis is not known.
|
The theca interna is composed of differentiated TICs located within a matrix of loose connective tissue and blood vessels (Fig. 19). In all graafian follicle, LH is a key regulatory hormone for TIC function, and its importance in regulating TIC androgen production in vivo and in vitro has been established.27 Beginning at the very early stages of graafian follicle development, the TICs express their differentiated state as androgen (i.e. androstenedione-producing cells).27 The theca interna is richly vascularized and serves to deliver hormones (e.g. FSH, LH), nutrient molecules, vitamins, and cofactors required for the growth and differentiation of the oocyte and granulosa cells.
We know little about the regulatory elements that control the theca vasculature. A functional link between the vasculature and graafian follicle development is suggested by the evidence43 that all monkey graafian follicles express high levels of FSH and LH receptor regardless of size, but when 125I-human chorionic gonadotropin (hCG) is injected systemically, only the dominant graafian follicle appears capable of accumulating 125I-hCG in the theca interna. These results suggest that the dominant graafian follicle expresses increased vascularization, which plays an important role in its selected maturation. In this regard, follicle-derived vascular endothelial growth factor44,45 and other angiogenic factors such as endothelin46 are being intensively investigated.
The theca compartments (i.e. theca externa and interna) express their differentiated functions at the beginning of graafian follicle development (at cavitation) and appear to constitutively express a mature phenotype throughout the life and death of the graafian follicle. In a broad sense, there is little or no evidence that major changes occur in the theca layers during the various stages of graafian follicle development beyond those related to vascular and proliferative activities. This could imply that it is the granulosa cells (and perhaps the oocyte) that are variable and therefore responsible for graafian follicle diversity.
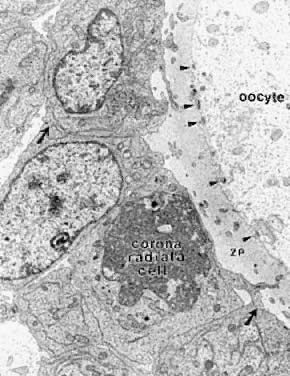
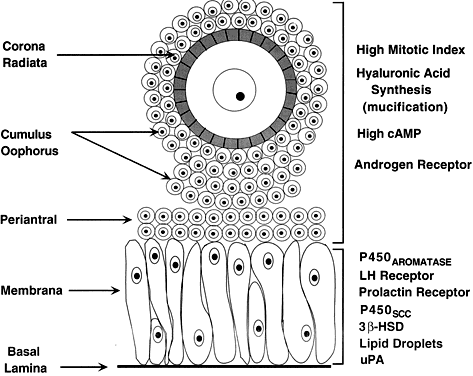
In the graafian follicle, the granulosa cells and oocyte exist as a mass of precisely shaped and precisely positioned cells (Fig. 18). The spatial variation creates at least four different granulosa cell layers or domains: the outermost domain is the membrana granulosa, the inner most domain is the periantral, the intermediate domain is the cumulus oophorus, and the domain juxtaposed to the oocyte is the corona radiata (Fig. 20). A characteristic histologic property of the membrana domain is that it is composed of a pseudostratified epithelium of tall columnar granulosa cells, all of which are anchored to the basal lamina.
The differentiation of a granulosa cell can be traced to its position within the cellular mass (Fig. 20). For example, cells in the membrana domain stop proliferating before those in central domain.47,48 The ability of the granulosa cells in the inner domains to continue dividing throughout graafian follicle development suggests they may be precursor cells. The cessation of mitosis in the membrana domain is characterized by the progressive expression of overt differentiation in which they assume the functional phenotype of fully differentiated cells. This process requires the temporal and coordinate expression of genes that form the basis of granulosa cytodifferentiation. The mechanisms by which this occurs involves ligand-dependent signaling pathways that are coupled to the activation and inhibition of specific genes. For example, normal differentiation of the membrana granulosa cells requires the activation of specific genes, including those for cytochrome P450 aromatase (P450arom)49 and the LH receptor,50 and the inhibition of structural genes in the apoptotic pathway. In contrast, the granulosa cells in the periantral, cumulus, and corona radiata domains proliferate, but they fail to express the genes that are involved in a terminal differentiation (Fig. 20).
What controls granulosa heterogeneity? All the granulosa cells in the healthy graafian follicle express FSH receptor,13,51,52 and it has been shown that murine granulosa cells in the membrana and cumulus domains produce cAMP in response to FSH stimulation.53 These observations argue that post cAMP regulatory events are involved in the aspects of granulosa heterogeneity. The idea that the oocyte plays a key role in causing the different patterns of granulosa cytodifferentiation during graafian follicle development is supported by studies in rodents.54 A dialogue takes place between the oocyte and granulosa cells that has a great impact on folliculogenesis. In developing murine graafian follicles, the differential pattern of proliferation and differentiation between the granulosa in the membrana and cumulus domains are under the control of secreted oocyte morphogens.54 A novel TGF-β family member, GDF-9, was discovered in the mouse.24,25 Definitive evidence that GDF-9 is obligatory for folliculogenesis came from studies of GDF-9-deficient mice.26 In these animals, the absence of GDF-9 resulted in the arrest of follicle growth and development at primary stage and the females are infertile. These data support the idea that GDF-9 secreted by the egg is obligatory for graafian follicle development, granulosa cytodifferentiation and proliferation, and female fertility. The clinical relevance of this new concept is demonstrated by the presence of GDF-9 mRNA in the human ovary.25 The current challenges are to elucidate the mechanisms controlling GDF-9 expression and to identify the target cells for GDF-9 and the biologic processes that GDF-9 regulates. The concept that oocyte-derived growth factors control folliculogenesis and fertility could have important implications for human physiology and pathophysiology.
Classification.
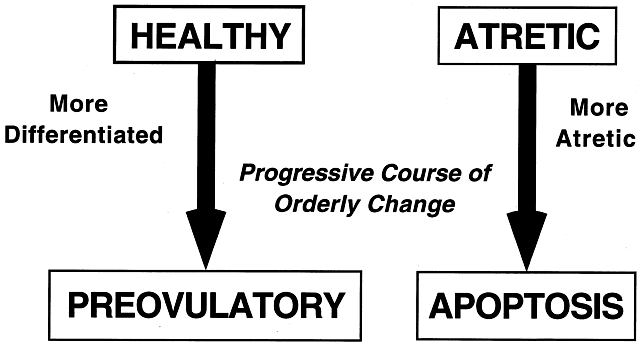
All graafian follicles can be divided broadly into two groups: healthy and atretic (Fig. 21). The main difference between these two groups is whether apoptosis is occurring in the granulosa cells. The development of a graafian follicle (healthy or atretic) follows a progressive course over time. This implies that variability or heterogeneity is a normal consequence of folliculogenesis. A healthy graafian follicle becomes progressively more differentiated with increasing time until it attains the preovulatory stage (Fig. 22). The time for this process (Fig. 2) is about 2 months in women.3 As this occurs, there is a temporal and spatial pattern of expression of large numbers of genes. In healthy follicles, these genes direct cytodifferentiation, proliferation, and follicular fluid formation. In atretic follicles, the time-dependent changes in gene expression cause the cessation of mitosis and the expression of apoptosis (i.e. follicle atresia). During atresia, the oocyte and granulosa cells become committed to express genes that lead to apoptosis.55 In healthy and atretic graafian follicles, the control mechanisms involve ligand-dependent signaling pathways that inhibit or stimulate the expression of differentiation and apoptosis (Fig. 22). Understanding the molecular mechanisms and cellular consequences of the ligand-receptor signaling pathways that control graafian follicle fate is a major goal of reproductive research.
The process of graafian follicle growth and development can be arbitrarily divided into several stages based on follicle size (Figs. 2 and 22). It is convenient and important for clinicians and researchers to identify the physiologic function of various types or classes of follicles over the cycle. The healthy human graafian follicle has a destiny to complete the transition from the small (1 to 6 mm), medium (7 to 11 mm), and large (12 to 17 mm) to the fully differentiated preovulatory state (18 to 23 mm). The atretic graafian follicle has a destiny to complete the transition from the small to the medium stage (1 to 10 mm) but appears incapable of growing to the large size under normal physiologic conditions.56 Because the process of graafian follicle development is asynchronous, it produces a large, heterogeneous population of graafian follicles in the ovaries at any moment in time (Fig. 3). Each of these morphologically distinct graafian follicles is a dynamic structure undergoing a flow or progression of developmental change on its way to becoming more differentiated or more atretic (Fig. 22). It should be kept in mind that this results in the presence of an extremely heterogeneous pool of graafian follicles. It is the heterogeneity that makes it difficult to come to grips with a simple functional definition for the graafian follicle.
The size of a graafian follicle is determined largely by the size of the antrum, which is determined by the volume of follicular fluid, which is determined by the bioavailability of FSH in the fluid.57 FSH is obligatory for graafian follicle development, and no other ligand by itself has the ability to induce follicular fluid formation. In the absence of FSH, follicular fluid is not produced, and no graafian follicles develop. The proliferation of the follicle cells also contributes to graafian follicle growth; in healthy follicles, the granulosa and theca cells proliferate extensively (as much as 100-fold), concomitant with the antrum becoming filled with follicular fluid (Fig. 23). These events (i.e. increased follicular fluid accumulation and cell proliferation) are responsible for the tremendous growth of healthy graafian follicles.3,58 In contrast, it is the cessation of mitosis and follicular fluid formation that determines the size of the atretic graafian follicle.
Selection of the Dominant Follicle.
In each menstrual cycle, the ovaries normally produce a single dominant follicle that participates in a single ovulation. Morphometric analysis of normal human ovaries (Figs. 2 and 3) indicates that the dominant follicle that will ovulate in the subsequent cycle is selected from a cohort of healthy, class 5 follicles measuring 4.7 ± 0.7 mm in diameter at the end of the luteal phase of the menstrual cycle.1,2,3,59 At the time of selection, each cohort follicle contains a fully grown oocyte, about 1 million granulosa cells, a theca interna containing several layers of TICs, and theca externa composed of smooth muscle cells (Figs. 3 and 23).
A characteristic feature of a dominant follicle is a high rate of mitosis in the granulosa cells. The evidence suggests that shortly after the mid-luteal phase, the rate of granulosa mitosis increases sharply (about twofold) in the granulosa cells within all cohort follicles.2,56,60 This suggests that luteolysis may be accompanied by a burst of mitosis in the granulosa of the cohort of class 5 follicles. The first indication that one follicle has been selected appears to be that the granulosa cells in the chosen follicle continue dividing at a relatively fast rate while proliferation slows in the granulosa of the other cohort follicles. Because this difference becomes apparent at the end of the luteal phase, it has been argued that selection occurs at the late luteal phase of the menstrual cycle. As a consequence of increased mitosis, the dominant follicle continues to grow rapidly3,4 during the follicular phase, reaching 6.9 ± 0.5 mm at days 1 to 5, 13.7 ± 1.2 mm at days 6 to 10, and 18.8 ± 0.5 mm at days 11 to 14. Conversely, growth proceeds more slowly in the cohort follicles, and with time, atresia becomes increasingly more evident in the nondominant cohort follicles, presumably because of the expression of specific genes in the apoptotic pathway.56 Rarely does an atretic follicle reach more than 10 mm in diameter, regardless of the stage in the cycle.4,56,60
The Process.
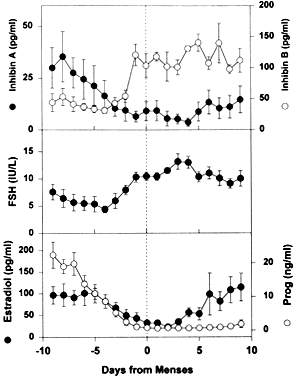
There is compelling evidence from laboratory animal61 and primate experiments,62 that a secondary rise in plasma FSH must be attained for a follicle to achieve dominance. As shown in Figure 24, the secondary FSH rise in women begins a few days before the progesterone levels fall to basal levels at the end of luteal phase, and the FSH levels remain elevated during the first week of the follicular phase of the cycle.63 Experiments using monkeys have demonstrated that the dominant follicle undergoes atresia if the secondary rise in FSH is prevented by treatment with exogenous estradiol.64 An important concept in reproductive biology is that an increase in bioactive FSH is obligatory for follicle selection and fertility.33,65 It appears that decreased estradiol production by the corpus luteum is the principal cause for the secondary rise in FSH66 rather than the fall in corpus luteum-derived inhibin A (Fig. 24).
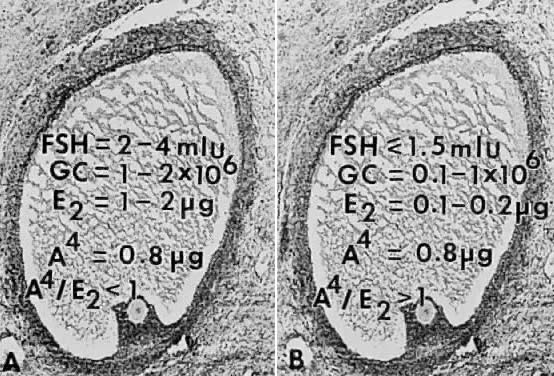
How does the secondary rise in FSH control selection? The results from studies of human follicular fluid support the conclusion that the rise in plasma FSH leads to a progressive accumulation of relatively high concentrations of FSH in the microenvironment of one follicle in the cohort; this follicle is destined to become dominant (Fig. 25). In developing healthy (dominant) follicles (class 5 to 8 follicles), the mean concentration of follicular fluid FSH increases from about 1.3 mIU/ml (about 58 ng/ml) to about 3.2 mIU/ml (about 143 ng/ml) through the follicular phase.4,67 In contrast,4,67 the levels of FSH are low or undetectable in the microenvironment of the nondominant cohort follicles (Fig. 25).
The entry of FSH into follicular fluid at cavitation is believed to provide the induction stimulus that initiates the process of graafian follicle growth and development. At the cellular level, it is the FSH receptor on the granulosa cell that is the fundamental player in this process. When an appropriate high FSH threshold is reached in one graafian follicle, it is selected to become dominant.31 In contrast, the small graafian follicles in the cohort with subthreshold levels of FSH become nondominant (Figs. 22 and 25). The mechanism whereby one small graafian follicle in a cohort is able to concentrate high levels of FSH in its microenvironment remains one of the mysteries in ovary physiology. An important point is that estradiol produced by the dominant follicle inhibits the secondary rise in FSH by a negative feedback mechanism (Figs. 24 and 26). This is believed to ensure a subthreshold level of FSH in the nondominant cohort follicles, which then leads to atresia. Mitosis in granulosa cells of atretic cohort follicles can be stimulated by treatment with human menopausal gonadotropin (hMG) during the early follicular phase.59 If FSH levels are increased to threshold levels within the microenvironment, then nondominant follicles may be rescued from atresia. This phenomenon could have implications for the way in which exogenous FSH or hMG triggers the formation of multiple dominant follicles in women undergoing ovulation induction.
AUTOCRINOLOGY AND PARACRINOLOGY
There is no doubt that the ability of the ovary to produce a dominant follicle, which ovulates a fertilizable egg, is under the control of the endocrine system, most notably by the hormones FSH and LH. Anything that interferes directly or indirectly with the normal action of the gonadotropins can be expected to produce a condition leading to apoptosis and infertility.
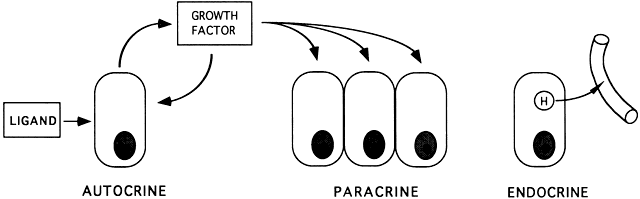
Research in the past decade has established the concept that FSH and LH signal transduction can be modulated by proteins with growth factor activity. All growth factors are ligands that act locally to amplify or attenuate cellular responses. The autocrine concept is that ligands (e.g. hormones, growth factors, neurotrophins, cytokines) produced by a cell act on the cell itself to modulate cellular activities (e.g. growth, differentiation, apoptosis) (Fig. 39). The paracrine concept is that ligands produced by one cell act on adjacent cells to modify or modulate cell functions (Fig. 39).
All five major families of growth factors are expressed within developing follicles of rats and humans.133 The principle emerging from an enormous amount of in vivo and in vitro research is that intrinsic growth factors interact with the endocrine system to evoke the physiologic control of all aspects of folliculogenesis, including recruitment, preantral follicle growth, selection, atresia, and ovulation. Two growth factors, oocyte-derived GDF-926 and granulosa-derived IGF-I,134 are obligatory for folliculogenesis and fertility in female mice.
The probability that new ovarian growth factor systems will be discovered in the future is high. Definitive evidence that local growth factors are obligatory for folliculogenesis and fertility in women is lacking, and the physiologic significance of the autocrine/paracrine concept in human ovaries remains to be established. The current challenges are to understand how specific autocrine/paracrine regulatory molecules control folliculogenesis and how these controls are integrated into the overall physiologic and pathophysiologic mechanisms.
ACKNOWLEDGMENTS
I thank Ms. Andi Hartgrove for typing the manuscript.
REFERENCES
Gougeon A: Dynamics of human follicular growth. In Adashi EY, Leung PCK (eds): The Ovary, p 21. New York: Raven Press, 1993 |
|
Gougeon A: Dynamics of follicular growth in the human: A model from preliminary results. Hum Reprod 1: 81, 1986 |
|
Gougeon A: Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17: 121, 1996 |
|
McNatty KP, Moore-Smith D, Osathanondh R, Ryan KJ: The human antral follicle: Functional correlates of growth and atresia. Ann Biol Anim Biochim Biophys 19: 1547, 1979 |
|
Gougeon A, Chainy GBN: Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil 81: 433, 1987 |
|
Reynolds SRM: The vasculature of the ovary and ovarian function. Recent Prog Horm Res 5: 65, 1950 |
|
Erickson GF: Basic biology: Ovarian anatomy and physiology. In Lobo R, Marcus R, Kelsey J (eds): Menopause. San Diego: Academic Press (in press) |
|
Erickson GF: The ovary: Basic principles and concepts. In Felig P, Frohman L (eds): Endocrinology and Metabolism. 4th ed. New York: McGraw Hill (in press) |
|
Faddy MJ, Gosden RG, Gougeon A et al: Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum Reprod 7: 1342, 1992 |
|
Gougeon A, Ecochard R, Thalabard JC: Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod 50: 653, 1994 |
|
Wandji SA, Srsen V, Nathanielsz PW et al: Initiation of growth of baboon primordial follicles in vitro. Hum Reprod 12: 1993, 1997 |
|
Henderson SA, Edwards RG: Chiasma frequency and maternal age in mammals. Nature 217: 22, 1968 |
|
Yamoto M, Shima K, Nakano R: Gonadotropin receptors in human ovarian follicles and corpora lutea throughout the menstrual cycle. Horm Res 37 (Suppl 1): 5, 1992 |
|
Oktay K, Briggs D, Gosden RG: Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab 82: 3748, 1997 |
|
Nakamura M, Minegishi T, Hasegawa Y et al: Effect of an activin A on follicle-stimulating hormone (FSH) receptor messenger ribonucleic acid levels and FSH receptor expressions in cultured rat granulosa cells. Endocrinology 133: 538, 1993 |
|
Bachvarova R: Gene expression during oogenesis and oocyte development in mammals. In Browder L (ed): Developmental Biology: A Comprehensive Synthesis, p 453. New York: Plenum, 1985 |
|
Wassarman PM, Liu C, Litscher ES: Constructing the mammalian egg zona pellucida: some new pieces of an old puzzle. J Cell Sci 109: 2001, 1996 |
|
Gong X, Dubois DH, Miller DJ, Shur BD: Activation of a G protein complex by aggregation of beta-1,4-galactosyltransferase on the surface of sperm. Science 269: 1718, 1995 |
|
Wassarman PM: Fertilization in mammals. Sci Am 259: 78, 1988 |
|
Beyer EC: Gap junctions. Int Rev Cytol 137C: 1, 1993 |
|
Kumar NM, Gilula NB: The gap junction communication channel. Cell 84: 381, 1996 |
|
Simon AM, Goodenough DA, Li E, Paul DL: Female infertility in mice lacking connexin 37. Nature 385: 525, 1997 |
|
Beyer EC, Kistler J, Paul DL, Goodenough DA: Antisera directed against connexin-43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol 108: 595, 1989 |
|
McPherron AC, Lee S-J: GDF-3 and GDF-9: Two new members of the transforming growth factor-β superfamily containing a novel pattern of cysteines. J Biol Chem 268: 3444, 1993 |
|
McGrath SA, Esquela AF, Lee S-J: Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol 9: 131, 1995 |
|
Dong J, Albertini DF, Nishimori K et al: Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383: 531, 1996 |
|
Erickson GF, Magoffin DA, Dyer C, Hofeditz C: The ovarian androgen producing cells: A review of structure/function relationships. Endocr Rev 6: 371, 1985 |
|
Eppig JJ: Regulation of mammalian oocyte maturation. In Adashi EY, Leung PCK (eds): The Ovary, p 185. New York: Raven Press, 1993 |
|
Eppig JJ: Oocyte-somatic cell communication in the ovarian follicles of mammals. Semin Dev Biol 5: 51, 1994 |
|
Erickson GF: Analysis of follicle development and ovum maturation. Semin Reprod Endocrinol 4: 233, 1986 |
|
Erickson GF: The graafian follicle: A functional definition. In Adashi EY (ed): Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer-Verlag (in press) |
|
Erickson GF: Primary cultures of ovarian cells in serum-free medium as models of hormone-dependent differentiation. Mol Cell Endocrinol 29: 21, 1983 |
|
Kumar TR, Wang Y, Lu N, Matzuk MM: Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15: 201, 1997 |
|
Kumar TR, Low MJ, Matzuk MM: Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology 139: 3289, 1998 |
|
Li R, Phillips DM, Mather JP: Activin promotes ovarian follicle development in vitro. Endocrinology 136: 849, 1995 |
|
Yoshida H, Takakura N, Kataoka H et al: Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol 184: 122, 1997 |
|
Edwards RG: Follicular fluid. J Reprod Fertil 37: 189, 1974 |
|
Amsterdam A, Lindner HR, Groschel-Stewart U: Localization of actin and myosin in the rat oocyte and follicular wall by immunofluorescence. Anat Res 187: 311, 1977 |
|
Self DA, Schroeder PC, Gown AM: Hamster thecal cells express muscle characteristics. Biol Reprod 39: 119, 1988 |
|
Motta PM, Familiari G: Occurrence of a contractile tissue in the theca externa of atretic follicles in the mouse ovary. Acta Anat 109: 103, 1981 |
|
Martin GG, Talbot P: The role of follicular smooth muscle cells in hamster ovulation. J Exp Zool 216: 469, 1981 |
|
Crisp TM, Dessouky DA, Denys FR: The fine structure of the human corpus luteum of early pregnancy and during the progestational phase of the menstrual cycle. Am J Anat 127: 37, 1970 |
|
Zeleznik AJ, Schuler HM, Reichert LE: Gonadotropin-binding sites in the rhesus monkey ovary: Role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology 109: 356, 1981 |
|
Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB: Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation. J Clin Endocrinol Metab 81: 353, 1996 |
|
Laitinen M, Ristimäki A, Honkasalo M et al: Differential hormonal regulation of vascular endothelial growth factors VEGF, VEGF-B, and VEGF-C messenger ribonucleic acid levels in cultured human granulosa-luteal cells. Endocrinology 138: 4748, 1997 |
|
Mancina R, Barni T, Calogero AE et al: Identification, characterization, and biological activity of endothelin receptors in human ovary. J Clin Endocrinol Metab 82: 4122, 1997 |
|
Hirshfield AN: Patterns of [3 H] thymidine incorporation differ in immature rats and mature, cycling rats. Biol Reprod 34: 229, 1986 |
|
Hirshfield AN: Granulosa cell proliferation in very small follicles of cycling rats studied by long-term continuous tritiated-thymidine infusion. Biol Reprod 41: 309, 1989 |
|
Whitelaw PF, Smyth CD, Howles CM, Hillier SG: Cell-specific expression of aromatase and LH receptor mRNAs in rat ovary. J Mol Endocrinol 9: 309, 1992 |
|
Bortolussi M, Marini G, Reolon ML: A histochemical study of the binding of 125 I-HCG to the rat ovary throughout the estrous cycle. Cell Tissue Res 197: 213, 1979 |
|
Camp TA, Rahal JO, Mayo KE: Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol 5: 1405, 1991 |
|
Minegishi T, Tano M, Igarashi M et al: Expression of follicle-stimulating hormone receptor in human ovary. Eur J Clin Invest 27: 469, 1997 |
|
Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ: FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 138: 16, 1990 |
|
Eppig JJ, Chesnel F, Hirao Y et al: Oocyte control of granulosa cell development: How and why. Hum Reprod 12: 127, 1997 |
|
Hsueh AJ, Billig H, Tsafriri A: Ovarian follicle atresia: A hormonally controlled apoptotic process. Endocr Rev 15: 707, 1994 |
|
Gougeon A, Lefèvre B: Evolution of the diameters of the largest healthy and atretic follicles during the human menstrual cycle. J Reprod Fertil 69: 497, 1983 |
|
Erickson GF: The ovary: Basic principles and concepts. In Felig P, Baxter JD, Frohman LA (eds): Endocrinology and Metabolism, p 973. 3rd ed. New York: McGraw-Hill, 1995 |
|
McNatty KP: Hormonal correlates of follicular development in the human ovary. Aust J Biol Sci 34: 249, 1981 |
|
Gougeon A, Testart J: Influence of human menopausal gonadotropin on the recruitment of human ovarian follicles. Fertil Steril 54: 848, 1990 |
|
Gougeon A: Qualitative changes in medium and large antral follicles in the human ovary during the menstrual cycle. Ann Biol Anim Biochem Biophys 19: 1461, 1979 |
|
Hirshfield AN: Development of follicles in the mammalian ovary. Int Rev Cytol 124: 43, 1991 |
|
Zeleznik AJ: Dynamics of primate follicular growth: A physiologic perspective. In Adashi EY, Leung PCK (eds): The Ovary, p 41. New York: Raven Press, 1993 |
|
Welt CK, Martin KA, Taylor AE et al: Frequency modulation of follicle-stimulating hormone (FSH) during the luteal-follicular transition: evidence for FSH control of inhibin B in normal women. J Clin Endocrinol Metab 82: 2645, 1997 |
|
Zeleznik AJ: Premature elevation of systemic estradiol reduces serum levels of follicle-stimulating hormone and lengthens the follicular phase of the menstrual cycle in rhesus monkeys. Endocrinology 109: 352, 1981 |
|
Matthews CH, Borgato S, Beck-Peccoz P et al: Primary amenorrhea and infertility due to a mutation in the beta-subunit of follicle-stimulating hormone. Nat Genet 5: 83, 1993 |
|
Lahlou N, Chabbert-Buffet N, Christin-Maitre S et al: Main inhibitor of follicle stimulating hormone in the luteal-follicular transition: inhibin A, oestradiol, or inhibin B? Hum Reprod 14: 1190, 1999 |
|
McNatty KP, Hunter WM, McNeilly AS, Sawers RS: Changes in the concentration of pituitary and steroid hormones in the follicular fluid of human graafian follicles throughout the menstrual cycle. J Endocrinol 64: 555, 1975 |
|
Simoni M, Gromoll J, Nieschlag E: The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18: 739, 1997 |
|
Ulloa-Aguirre A, Midgley AR Jr, Beitins IZ, Padmanabhan V: Follicle-stimulating isohormones: characterization and physiological relevance. Endocr Rev 16: 765, 1995 |
|
Davis D, Liu X, Segaloff DL: Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in FSH receptor function. Mol Endocrinol 9: 159, 1995 |
|
Gromoll J, Gudermann T, Nieschlag E: Molecular cloning of a truncated isoform of the human follicle stimulating hormone receptor. Biochem Biophys Res Commun 188: 1077, 1992 |
|
Meyer TE, Habener JF: Cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acidbinding proteins. Endocr Rev 14: 269, 1993 |
|
McNatty KP, Sawers RS: Relationship between the endocrine environment within the graafian follicle and the subsequent rate of progesterone secretion by human granulosa cells in vitro. J Endocrinol 66: 391, 1975 |
|
Yong EL, Baird DT, Hillier SG: Mediation of gonadotrophin-stimulated growth and differentiation of human granulosa cells by adenosine-3',5'-monophosphate: one molecule, two messages. Clin Endocrinol (Oxf) 37: 51, 1992 |
|
Yong EL, Baird DT, Yates R et al: Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab 74: 842, 1992 |
|
Gospodarowicz D, Bialecki H: Fibroblast and epidermal growth factors are mitogenic agents for cultured granulosa cells of rodent, porcine, and human origin. Endocrinology 104: 757, 1979 |
|
Delforge JP, Thomas K, Roux F et al: Time relationships between granulosa cells growth and luteinization, and plasma luteinizing hormone discharge in human. 1. A morphometric analysis. Fertil Steril 23: 1, 1972 |
|
Channing CP: Influences of the in vivo and in vitro hormonal environment upon luteinization of granulosa cells in tissue culture. Recent Prog Horm Res 26: 589, 1970 |
|
Steinkampf MP, Mendelson CR, Simpson ER: Regulation by follicle-stimulating hormone of the synthesis of aromatase cytochrome P-450 in human granulosa cells. Mol Endocrinol 1: 465, 1987 |
|
Sawatawan C, Milewich L, Word RA et al: Compartmentalization of type I 17 beta-hydroxysteroid oxidoreductase in the human ovary. Mol Cell Endocrinol 99: 161, 1994 |
|
Ghersevich SA, Poutanen MH, Martikainen HK, Vihko RK: Expression of 17 beta-hydroxysteroid dehydrogenase in human granulosa cells: Correlation with follicular size, cytochrome P450 aromatase activity and oestradiol production. J Endocrinol 143: 139, 1994 |
|
Zhang Y, Word RA, Fesmire S et al: Human ovarian expression of 17 beta-hydroxysteroid dehydrogenase types 1, 2, and 3. J Clin Endocrinol Metab 81: 3594, 1996 |
|
Inkster SE, Brodie AM: Expression of aromatase cytochrome P-450 in premenopausal and postmenopausal human ovaries: An immunocytochemical study. J Clin Endocrinol Metab 73: 717, 1991 |
|
Suzuki T, Sasano H, Tamura M et al: Temporal and spatial localization of steroidogenic enzymes in premenopausal human ovaries: in situ hybridization and immunohistochemical study. Mol Cell Endocrinol 97: 135, 1993 |
|
Doody KJ, Lorence MC, Mason JI, Simpson ER: Expression of messenger ribonucleic acid species encoding steroidogenic enzymes in human follicles and corpora lutea throughout the menstrual cycle. J Clin Endocrinol Metab 70: 1041, 1990 |
|
Kiriakidou M, McAllister JM, Sugawara T, Strauss JFR: Expression of steroidogenic acute regulatory protein STAR in the human ovary. J Clin Endocrinol Metab 81: 4122, 1996 |
|
Voutilainen R, Tapanainen J, Chung BC et al: Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab 63: 202, 1986 |
|
Penning TM: Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev 18: 281, 1997 |
|
Shimasaki S, Zachow RJ, Li D et al: A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci USA 96; 7282, 1999 |
|
Richards JS: Hormonal control of gene expression in the ovary. Endocr Rev 15: 725, 1994 |
|
Erickson GF, Wang C, Hsueh AJW: FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature 279: 336, 1979 |
|
Shaw HJ, Hillier SG, Hodges JK: Developmental changes in luteinizing hormone/human chorionic gonadotropin steroidogenic responsiveness in marmoset granulosa cells: Effects of follicle-stimulating hormone and androgens. Endocrinology 124: 1669, 1989 |
|
Bar-Ami S, Haciski RC, Channing CP: Increasing 125 I-human chorionic gonadotrophin specific binding in human granulosa cells by follicle-stimulating hormone and follicular fluid. Hum Reprod 4: 876, 1989 |
|
Yong EL, Hillier SG, Turner M et al: Differential regulation of cholesterol side-chain cleavage P450scc and aromatase P450arom enzyme mRNA expression by gonadotrophins and cyclic AMP in human granulosa cells. J Mol Endocrinol 12: 239, 1994 |
|
Enmark E, Pelto-Huikko M, Grandien K et al: Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 82: 4258, 1997 |
|
El-Roeiy A, Chen X, Roberts VJ et al: Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab 77: 1411, 1993 |
|
Pollack SE, Furth EE, Kallen CB et al: Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metab 82: 4243, 1997 |
|
Tamura T, Kitawaki J, Yamamoto T et al: Immunohistochemical localization of 17α-hydroxylase/C17-20 lyase and aromatase cytochrome P-450 in polycystic human ovaries. J Endocrinol 139: 503, 1993 |
|
Erickson GF: Normal regulation of ovarian androgen production. Semin Reprod Endocrinol 11: 307, 1993 |
|
Erickson GF: Ovarian androgen biosynthesis: Endocrine regulation. In Azziz R, Nestler JE, Dewailly D (eds): Androgen Excess Disorders in Women, p 3. New York: Lippincott-Raven Publishers, 1997 |
|
Erickson GF, Magoffin DA, Jones KL: Theca function in polycystic ovaries of a patient with virilizing congenital adrenal hyperplasia. Fertil Steril 51: 173, 1989 |
|
Erickson GF, Magoffin DA, Dyer CA, Hofeditz C: The ovarian androgen producing cells: A review of structure/function relationships. Endocr Rev 6: 371, 1985 |
|
Magoffin DA, Erickson GF: Control systems of theca-interstitial cells. In Findlay JK (ed): Molecular Biology of the Female Reproductive System, p 39. New York: Academic Press, 1994 |
|
Toledo SP, Brunner HG, Kraaij R et al: An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46,XX female. J Clin Endocrinol Metab 81: 3850, 1996 |
|
Erickson GF: PCO: The ovarian connection. In Adashi EY, Rock JA, Rosenwaks Z (eds): Reproductive Endocrinology, Surgery, and Technology, p 1141. New York: Raven Press, 1995 |
|
Segaloff DL, Ascoli M: The lutropin/choriogonadotropin receptor … 4 years later. Endocr Rev 14: 324, 1993 |
|
Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ: Phosphorylation of cell surface receptors: A mechanism for regulating signal transduction pathways. Endocr Rev 9: 38, 1988 |
|
Barbieri RL, Smith S, Ryan KJ: The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil Steril 50: 197, 1988 |
|
Poretsky L: On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev 12: 3, 1991 |
|
Legro RS, Spielman R, Urbanek M et al: Phenotype and genotype in polycystic ovary syndrome. Recent Prog Horm Res 53: 217, 1998 |
|
Hillier SG, Yong EL, Illingworth PJ et al: Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J Clin Endocrinol Metab 72: 1206, 1991 |
|
Hillier SG, Yong EL, Illingworth PJ et al: Effect of recombinant inhibin on androgen synthesis in cultured human thecal cells. Mol Cell Endocrinol 75: R1, 1991 |
|
Garzo VG, Dorrington JH: Aromatase activity in human granulosa cells during follicular development and the modulation by follicle-stimulating hormone and insulin. Am J Obstet Gynecol 148: 657, 1984 |
|
Erickson GF, Garzo VG, Magoffin DA: Insulin-like growth factor 1 (IGF-1) regulates aromatase activity in human granulosa and granulosa luteal cells. J Clin Endocrinol Metab 69: 716, 1989 |
|
Steinkampf MP, Mendelson CR, Simpson ER: Effects of epidermal growth factor and insulin-like growth factor I on the levels of mRNA encoding aromatase cytochrome P-450 of human ovarian granulosa cells. Mol Cell Endocrinol 59: 93, 1988 |
|
Rice VM, Williams VR, Limback SD, Terranova PF: Tumour necrosis factor-alpha inhibits follicle-stimulating hormone-induced granulosa cell oestradiol secretion in the human: Dependence on size of follicle. Hum Reprod 11: 1256, 1996 |
|
Tilly JL: Apoptosis and ovarian function. Rev Reprod 1: 162, 1996 |
|
Erickson GF: Defining apoptosis: Players and systems. J Soc Gynecol Invest 4: 219, 1997 |
|
Wyllie AH: Cell death: The significance of apoptosis. Int Rev Cytol 68: 251, 1980 |
|
Ashkenazi A, Dixit VM: Death receptors: Signaling and modulation. Science 281: 1305, 1998 |
|
Green DR, Reed JC: Mitochondria and apoptosis. Science 281: 1309, 1998 |
|
Thornberry NA, Lazebnik Y: Caspases: enemies within. Science 281: 1312, 1998 |
|
Evan G, Littlewood T: A matter of life and cell death. Science 281: 1317, 1998 |
|
Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322, 1998 |
|
Tilly JL, Tilly KI, Perez GI: The genes of cell death and cellular susceptibility to apoptosis in the ovary: A hypothesis. Cell Death Differ 4: 180, 1997 |
|
Tsafriri A, Chun SY, Reich R: Follicular rupture and ovulation. In Adashi EY, Leung PCK (eds): The Ovary, p 227. New York: Raven Press, 1993 |
|
Espey LL: Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod 50: 233, 1994 |
|
Takahashi T, Ohnishi J: Molecular mechanism of follicle rupture during ovulation. Zoolog Sci 12: 359, 1995 |
|
Lydon JP, DeMayo FJ, Funk CR et al: Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9: 2266, 1995 |
|
Dinchuk JE, Car BD, Focht RJ et al: Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406, 1995 |
|
Lim H, Paria BC, Das SK et al: Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91: 197, 1997 |
|
Beers WH: Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell 6: 379, 1975 |
|
Adashi E, Leung PCK (eds): The Ovary: Comprehensive Endocrinology. New York: Raven Press, 1993 |
|
Baker J, Hardy MP, Zhou J et al: Effects of an lgfl gene null mutation on mouse reproduction. Mol Endocrinol 10: 903, 1996 |



 300 days for a recruited primordial to grow and develop to the class 2/3 (0.4 mm) or cavitation (early antrum) stage. Atresia can occur in preantral class 1, 2, and 3 follicles. Antral period: A class 4 (1 to 2 mm) follicle, if selected, requires about 50 days to grow and develop to the preovulatory stage. The dominant follicle of the cycle appears to be selected from a cohort of class five follicles, and it requires about 20 days to develop to the ovulatory stage. Atresia is common during the antral period. gc, number of granulosa cells; d, days.
300 days for a recruited primordial to grow and develop to the class 2/3 (0.4 mm) or cavitation (early antrum) stage. Atresia can occur in preantral class 1, 2, and 3 follicles. Antral period: A class 4 (1 to 2 mm) follicle, if selected, requires about 50 days to grow and develop to the preovulatory stage. The dominant follicle of the cycle appears to be selected from a cohort of class five follicles, and it requires about 20 days to develop to the ovulatory stage. Atresia is common during the antral period. gc, number of granulosa cells; d, days.